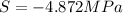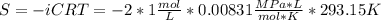Chemistry, 29.10.2019 21:31 tynyiaawrightt
Assuming that the nacl is completely ionized, calculate how much it will lower the solute potential of the soil at 20°c using the solute potential equation: ѱs = –icrt where i is the ionization constant (2 for nacl), c is the molar concentration (in mol/l), r is the pressure constant [r = 0.00831 l • mpa/(mol • k)], and t is the temperature in kelvin (273 + °c). how much will the solute potential of the soil be lowered at 20°c?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:20, lex68259100
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1


Chemistry, 22.06.2019 18:30, chinadoll24
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 22.06.2019 23:50, josie311251
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3
You know the right answer?
Assuming that the nacl is completely ionized, calculate how much it will lower the solute potential...
Questions in other subjects:

Physics, 23.03.2021 19:50

Physics, 23.03.2021 19:50


Mathematics, 23.03.2021 19:50



Mathematics, 23.03.2021 20:00


Mathematics, 23.03.2021 20:00

Physics, 23.03.2021 20:00