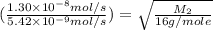Chemistry, 29.10.2019 19:31 Hippiekoolaid
Methane (ch4) effuses through a small opening in the side of a container at the rate of 1.30 × 10 8 mol s 1. an unknown gas effuses through the same opening at the rate of 5.42 × 10 9 mol s 1 when maintained at the same tem- perature and pressure as the methane. determine the molar mass of the unknown gas.

Answers: 1


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 22:30, COOLIOMARIS
What three things does a balanced equation show you?
Answers: 1
You know the right answer?
Methane (ch4) effuses through a small opening in the side of a container at the rate of 1.30 × 10 8...
Questions in other subjects:

English, 05.11.2020 01:00




Biology, 05.11.2020 01:00




English, 05.11.2020 01:00

Mathematics, 05.11.2020 01:00

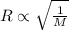
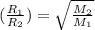 ..........(1)
..........(1) = rate of effusion of methane gas =
= rate of effusion of methane gas = 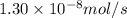
 = rate of effusion of unknown gas =
= rate of effusion of unknown gas = 
 = molar mass of methane gas = 16 g/mole
= molar mass of methane gas = 16 g/mole = molar mass of unknown gas = ?
= molar mass of unknown gas = ?