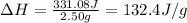Chemistry, 29.10.2019 07:31 acavalieri72
Consider the dissolution of 2.50 grams of salt xy in 75.0 ml of water within a calorimeter. the temperature of the water decreased by 0.93 oc. the heat capacity of the calorimeter is 42.2 j/oc. the density of the water (and the solution) is 1.00 g/ml. the specific heat capacity of the solution is 4.184 j/goc. calculate the enthalpy change for dissolving this salt on a energy per mass basis (units of j/g).

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, omoaye
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 10:10, alvaradolm6853
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 12:00, ctyrector
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2
You know the right answer?
Consider the dissolution of 2.50 grams of salt xy in 75.0 ml of water within a calorimeter. the temp...
Questions in other subjects:

Mathematics, 03.12.2021 02:40


Social Studies, 03.12.2021 02:40

Mathematics, 03.12.2021 02:40

Computers and Technology, 03.12.2021 02:40




Mathematics, 03.12.2021 02:40

Social Studies, 03.12.2021 02:40

![q=[q_1+q_2]](/tpl/images/0350/9166/341bc.png)
![q=[c_1\times \Delta T+m_2\times c_2\times \Delta T]](/tpl/images/0350/9166/1d50b.png)
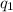 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter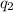 = heat absorbed by the water
= heat absorbed by the water = specific heat of calorimeter =
= specific heat of calorimeter = 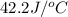
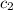 = specific heat of water =
= specific heat of water = 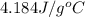
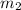 = mass of water =
= mass of water = 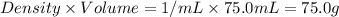
 = change in temperature =
= change in temperature = 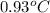
![q=[(42.2J/^oC\times 0.93^oC)+(75.0g\times 4.184J/g^oC\times 0.93^oC)]](/tpl/images/0350/9166/57473.png)
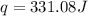
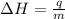
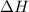 = enthalpy change = ?
= enthalpy change = ?