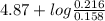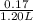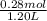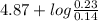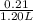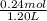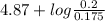Chemistry, 29.10.2019 05:31 stuckonquestions
Abuffer contains 0.19 mol of propionic acid (c2h5cooh) and 0.26 mol of sodium propionate (c2h5coona) in 1.20 l. you may want to reference (pages 721 - 729) section 17.2 while completing this problem. part a what is the ph of this buffer? express the ph to two decimal places. php h = nothing request answer part b what is the ph of the buffer after the addition of 0.02 mol of naoh? express the ph to two decimal places. php h = nothing request answer part c what is the ph of the buffer after the addition of 0.02 mol of hi? express the ph to two decimal places.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:20, jtingley0502
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 13:50, amandamac7339
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 21:00, agarcia24101993
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1
You know the right answer?
Abuffer contains 0.19 mol of propionic acid (c2h5cooh) and 0.26 mol of sodium propionate (c2h5coona)...
Questions in other subjects:


Chemistry, 08.03.2021 09:10


Mathematics, 08.03.2021 09:10

Mathematics, 08.03.2021 09:10


English, 08.03.2021 09:10

English, 08.03.2021 09:10


Mathematics, 08.03.2021 09:10

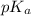 of propionic acid = 4.87
of propionic acid = 4.87
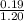
![pK_{a} + log(\frac{[salt]}{[acid]})](/tpl/images/0350/6880/fe481.png)