
Chemistry, 29.10.2019 04:31 21ghostrider21
The first step in the process used to recover zinc metal from zinc sulfide ore is the reaction of zinc sulfide with oxygen gas to produce zinc oxide and sulfur dioxide. 2zns(s) 3o2(g)⟶2zno(s) 2so2(g) when the external pressure is 1.523×105 pa and the temperature is 675 k, the amount of work performed is 850.1 j. calculate how many grams of oxygen are consumed in the reaction.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, bobbycisar1205
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 09:40, gonzaleze18
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 12:40, whitethunder05
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 15:30, lizzyhearts
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3
You know the right answer?
The first step in the process used to recover zinc metal from zinc sulfide ore is the reaction of zi...
Questions in other subjects:

Computers and Technology, 26.01.2021 20:10




Biology, 26.01.2021 20:10

Mathematics, 26.01.2021 20:10





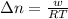
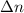 = change in moles of gas = ?
= change in moles of gas = ?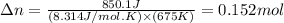
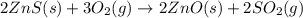
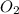 = 32 g/mol
= 32 g/mol

