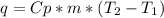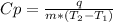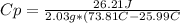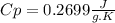Chemistry, 29.10.2019 01:31 thebrain1345
The amount of heat (q) gained or lost by a substance (with mass m) as its temperature changes (δt) depends on its specific heat capacity (cp) according to the following equation. q = cp ✕ m ✕ δt the quality of industrial diamonds is determined in part by measuring the specific heat capacity, which is 0.5091 j/g·k for pure diamond. if the absorption of 26.21 j of heat by a 2.03 g diamond sample of unknown purity causes its temperature to rise from 25.99°c to 73.81°c, is the diamond sample pure? explain your reasoning.

Answers: 1


Other questions on the subject: Chemistry



You know the right answer?
The amount of heat (q) gained or lost by a substance (with mass m) as its temperature changes (δt) d...
Questions in other subjects:


Chemistry, 23.10.2021 20:50

Biology, 23.10.2021 20:50

Mathematics, 23.10.2021 20:50

English, 23.10.2021 20:50

Mathematics, 23.10.2021 20:50

Mathematics, 23.10.2021 20:50

Chemistry, 23.10.2021 20:50


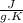 and it is different from the specific heat capacity found that is 0.2699
and it is different from the specific heat capacity found that is 0.2699