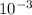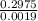Chemistry, 28.10.2019 23:31 crodriguez87
Astudent dissolves 0.2975 g of the unknown weak acid to give 100. ml solution. s/he obtains the following titration curve for his/her best titration. at the equivalence point, 19.0 ml of 0.1000 m naoh was added.(a) calculate the molar mass of the acid. g/mol(b) what is the pka of the acid?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, alexisgoss8091
Which traits do human embryos have that link them to a common ancestor with fish and reptiles? a. scales and tail b. gill slits and scales c. tail and gill slits d. hair and tail
Answers: 2

Chemistry, 22.06.2019 10:30, tjjjjjjjjjjjjjjjjjjj
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 12:40, carebear60
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

You know the right answer?
Astudent dissolves 0.2975 g of the unknown weak acid to give 100. ml solution. s/he obtains the foll...
Questions in other subjects:

Biology, 27.10.2021 06:20

History, 27.10.2021 06:20


Biology, 27.10.2021 06:20




Mathematics, 27.10.2021 06:20


World Languages, 27.10.2021 06:20

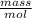 .....................1
.....................1