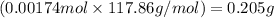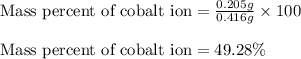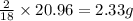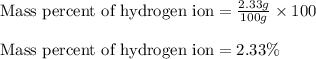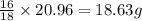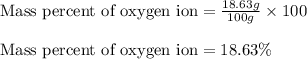In most of its ionic compounds, cobalt is either co(ii) or co(iii). one such compound, containing chloride ion and waters of hydration, was analyzed, and the following results were obtained. a 0.256-g sample of the compound was dissolved in water, and excess silver nitrate was added. the silver chloride was filtered, dried, and weighed, and it had a mass of 0.308 g. a second sample of 0.416 g of the compound was dissolved in water, and an excess of sodium hydroxide was added. the hydroxide salt was filtered and heated in a flame, forming cobalt(iii) oxide. the mass of the cobalt(iii) oxide formed was 0.145 g. what is the percent composition, by mass, of the compound?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, kayleg907436
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 02:40, hardwick744
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 14:50, rebeccamckellpidge
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 17:30, kaytonleeb
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2
You know the right answer?
In most of its ionic compounds, cobalt is either co(ii) or co(iii). one such compound, containing ch...
Questions in other subjects:

Biology, 16.10.2020 15:01

Social Studies, 16.10.2020 15:01





Mathematics, 16.10.2020 15:01



Mathematics, 16.10.2020 15:01

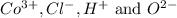 ions in the compound are 49.28 %, 29.79 %, 2.33 % and 18.63 % respectively
ions in the compound are 49.28 %, 29.79 %, 2.33 % and 18.63 % respectively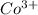 ions = 0.416 grams
ions = 0.416 grams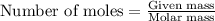 ......(1)
......(1)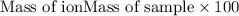 .......(2)
.......(2)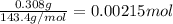
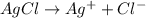
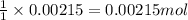 of chloride ions
of chloride ions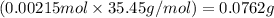
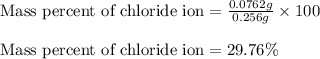
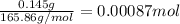
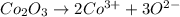
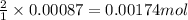 of cobalt ions
of cobalt ions