Chemistry, 28.10.2019 13:31 floreschachi8230
Fixed amount of gas at 25.0 °c occupies a volume of 10.0 l when the pressure is 667 torr. use boyle's law to calculate the pressure (torr) when the volume is reduced to 7.88 l at a constant temperature of 25.0°c

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:00, bernicewhite156
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

Chemistry, 22.06.2019 08:30, myamiller558
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 18:30, rosie20052019
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1
You know the right answer?
Fixed amount of gas at 25.0 °c occupies a volume of 10.0 l when the pressure is 667 torr. use boyle'...
Questions in other subjects:



Biology, 04.01.2021 08:30




Mathematics, 04.01.2021 08:30

History, 04.01.2021 08:30

Mathematics, 04.01.2021 08:30

Social Studies, 04.01.2021 08:30

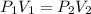
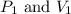 are initial pressure and volume of the gas.
are initial pressure and volume of the gas.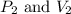 are final pressure and volume of the gas
are final pressure and volume of the gas