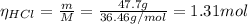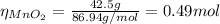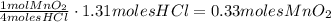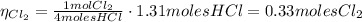Chemistry, 26.10.2019 04:43 jamaiciaw6
Chlorine gas can be prepared in the laboratory by the reaction of hydrochloric acid with manganese(iv) oxide:
4hcl(aq) + mno2(s) > mncl2(aq) + 2h2o(l) + cl2(g)
you add 42.5 g of mno2 to a solution containing 47.7 g of hcl.
(a) what is the limiting reactant? mno2 or hcl?
(b)what is the theortical yield of co2?
(c) if the yield of the reaction is 79.9%, what is the actual yield of chlorine?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, smhrosepetals
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 22.06.2019 04:10, tishfaco5000
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2


Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2
You know the right answer?
Chlorine gas can be prepared in the laboratory by the reaction of hydrochloric acid with manganese(i...
Questions in other subjects:

Social Studies, 28.01.2021 03:10






Mathematics, 28.01.2021 03:10

English, 28.01.2021 03:10

Mathematics, 28.01.2021 03:10