
Chemistry, 26.10.2019 03:43 barnhill6534
It turns out that the van dar waals constant b is equal to four times the total volume actually occupied by the molecules of a mole of gas. using this figure, calculate the fraction of the volume in a container actually occupied by ar atoms:
a) at stp
b) at 100 atm pressure and 0 degrees celsius

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:00, aleilyg2005
If two objects at different te, peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 13:00, jaylanmahone223
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 23:30, johnnysteeler9934
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1
You know the right answer?
It turns out that the van dar waals constant b is equal to four times the total volume actually occu...
Questions in other subjects:

Mathematics, 26.06.2019 19:30


English, 26.06.2019 19:30

Mathematics, 26.06.2019 19:30

Mathematics, 26.06.2019 19:30

Biology, 26.06.2019 19:30

Biology, 26.06.2019 19:30



Health, 26.06.2019 19:30

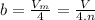 (1)
(1)



