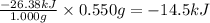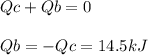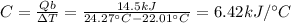Chemistry, 26.10.2019 02:43 holman9308
The combustion of exactly 1.000 g of benzoic acid in a bomb calorimeter releases 26.38 kj of heat. if the combustion of 0.550 g of benzoic acid causes the temperature of the calorimeter to increase from 22.01∘c to 24.27∘c, calculate the heat capacity of the calorimeter.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, 21brooklynmartin
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2

Chemistry, 22.06.2019 01:10, mistiehaas
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 02:40, gabrielolivas59
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 05:30, medlinalex
Compare and contrast physical changes with chemical changes.
Answers: 1
You know the right answer?
The combustion of exactly 1.000 g of benzoic acid in a bomb calorimeter releases 26.38 kj of heat. i...
Questions in other subjects:


History, 25.01.2022 01:30


Chemistry, 25.01.2022 01:30

Chemistry, 25.01.2022 01:30

Physics, 25.01.2022 01:30


Mathematics, 25.01.2022 01:30

Computers and Technology, 25.01.2022 01:30

Geography, 25.01.2022 01:30