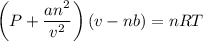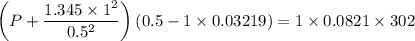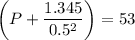Chemistry, 26.10.2019 01:43 meadowsoares7
If 1.00 mol of argon is placed in a 0.500-l container at 29.0 ∘c , what is the difference between the ideal pressure (as predicted by the ideal gas law) and the real pressure (as predicted by the van der waals equation)? for argon, a=1.345(l2⋅atm)/mol2 and b=0.03219l/mol.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, alexusnicole817
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 09:30, jewelz5887
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 12:00, zamariahyou
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 17:30, TheViperMlg23676
What causes most sediment to wash or fall into a river
Answers: 1
You know the right answer?
If 1.00 mol of argon is placed in a 0.500-l container at 29.0 ∘c , what is the difference between th...
Questions in other subjects:

Mathematics, 21.01.2022 08:00

Computers and Technology, 21.01.2022 08:00

Mathematics, 21.01.2022 08:00

English, 21.01.2022 08:00

SAT, 21.01.2022 08:00


English, 21.01.2022 08:00

English, 21.01.2022 08:00