
Chemistry, 26.10.2019 00:43 gizmo50245
Aspirin (c9h8o4) is synthesized by reacting salicylic acid (c7h6o3) with acetic anhydride (c4h6o3) according to the reaction: c7h6o3 + c4h6o3 --> c9h8o4 + hc2h3o2 a. what mass of acetic anhydride is needed to completely react 1.00 x 10 4 g of salicylic acid?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:50, chem1014
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 22:30, wpatskiteh7203
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1

Chemistry, 23.06.2019 01:00, liamgreene90
The time that is taken by neptune once around the sun is called
Answers: 1
You know the right answer?
Aspirin (c9h8o4) is synthesized by reacting salicylic acid (c7h6o3) with acetic anhydride (c4h6o3) a...
Questions in other subjects:







Physics, 25.01.2020 02:31



Computers and Technology, 25.01.2020 02:31

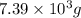 of acetic anhydride is needed
of acetic anhydride is needed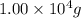 of salicylic acid =
of salicylic acid = 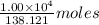 of salicylic acid
of salicylic acid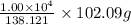 =
= 

