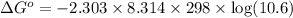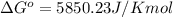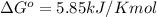Chemistry, 26.10.2019 00:43 noellelovebug1214
At 25 ∘c , the equilibrium partial pressures for the reaction were found to be pa=5.16 bar, pb=5.04 bar, pc=4.11 bar, and pd=4.85 bar . a(g)+2b(g)↽−−⇀4c(g)+d(g) what is the standard change in gibbs free energy of this reaction at 25 ∘c ? δ∘rxn= kjmol

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, stefaniethibodeaux
Which supports the idea that birds and butterflies both have wings but they do not have a common ancestor with wings? a. the wings are analogous structures that evolved differently and do not have a similar internal structure. b. the wings are homologous structures that evolved differently and do not have a similar internal structure. c. wings of birds are vestigial structures, but the wing structures of bats are not vestigial. d. wings of bats are vestigial structures, but the wing structures of birds are not vestigial
Answers: 1


Chemistry, 22.06.2019 17:30, llamasking
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 22.06.2019 17:40, Snowball080717
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3
You know the right answer?
At 25 ∘c , the equilibrium partial pressures for the reaction were found to be pa=5.16 bar, pb=5.04...
Questions in other subjects:


Mathematics, 17.07.2019 05:30

History, 17.07.2019 05:30

Biology, 17.07.2019 05:30

History, 17.07.2019 05:30

Mathematics, 17.07.2019 05:30

History, 17.07.2019 05:30


Mathematics, 17.07.2019 05:30

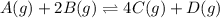
![K_p=\frac{[p_{D}]\times [p_{C}]}^4{[p_{B}]^2\times [p_{A}]}](/tpl/images/0346/8447/cda48.png)
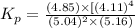
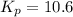
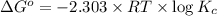
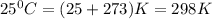
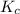 = equilibrium constant = 10.6
= equilibrium constant = 10.6