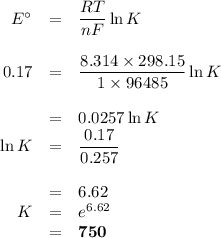Chemistry, 25.10.2019 22:43 michellemitchel
In the mitochondrial electron transfer chain, one of the steps that mediates transfer of electrons from nadh to o2 is the transfer of electrons from the reduced (ferrous) heme of cytochrome b to the oxidized (ferric) heme of cytochrome c1: fe ii (cyt b) + fe iii (cyt c1) → fe iii (cyt b) + fe ii (cyt c1) the standard state reduction potentials of fe iii (cyt c1) and fe iii (cyt b) are 0.25 v and 0.08 v, respectively, relative to the standard hydrogen electrode.(a) express the above redox reaction as two half reactions, one as an oxidation and one as a reduction. (b) determine the equilibrium constant for the reaction as written. is this reaction spontaneous, favoring products?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, willcohen42
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2

Chemistry, 21.06.2019 20:40, ellemarshall13
Which of the following pressures is equal to 760 mm hg? 2.0 atm 101.3 pa 101,300 kpa 101,300 pa
Answers: 2

You know the right answer?
In the mitochondrial electron transfer chain, one of the steps that mediates transfer of electrons f...
Questions in other subjects:


Chemistry, 02.02.2021 04:50

Mathematics, 02.02.2021 04:50






Biology, 02.02.2021 04:50