

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, suemmimonjaras8374
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1


Chemistry, 22.06.2019 15:00, emmalie52
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
A0.15 m solution of chloroacetic acid has a ph of 1.86. what is the value of ka for this acid?...
Questions in other subjects:

Mathematics, 09.03.2021 20:20

Mathematics, 09.03.2021 20:20

Mathematics, 09.03.2021 20:20

Mathematics, 09.03.2021 20:20

Mathematics, 09.03.2021 20:20

Mathematics, 09.03.2021 20:20

Mathematics, 09.03.2021 20:20



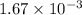
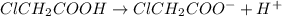
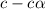
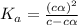
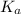 = ?
= ?![pH=-log[H^+]](/tpl/images/0346/5539/15713.png)
![1.86=-log[H^+]](/tpl/images/0346/5539/7260c.png)
![[H^+]=0.01](/tpl/images/0346/5539/6c620.png)
![[H^+]=c\times \alpha](/tpl/images/0346/5539/4fc41.png)
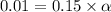
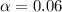
![[H^+]=[ClCH_2COO^-]=0.01](/tpl/images/0346/5539/98324.png)
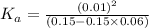
![K_a=1.67\times 10^{-3]](/tpl/images/0346/5539/1973d.png)


