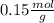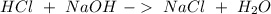Chemistry, 25.10.2019 20:43 alexisolermeador389
Astudent used 0.29 g of a sample and added to it 50.0 ml of 0.100 m hcl. the resulting solution then required 39.50 ml of 0.0980 m naoh to reach a ph of 3.0. what is the acid consuming capacity of the sample in moles of acid neutralized per gram of sample?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, happy121906
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 10:00, melissa9882
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1


Chemistry, 23.06.2019 00:20, destromero
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1
You know the right answer?
Astudent used 0.29 g of a sample and added to it 50.0 ml of 0.100 m hcl. the resulting solution then...
Questions in other subjects:



Mathematics, 26.10.2020 17:30