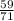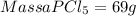Chemistry, 25.10.2019 20:43 babycakez143
What mass of pcl5 will be produced from the given masses of both reactants? 28.0 g of p4 and 59.0 g of cl2

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, haileywebb8
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 22.06.2019 00:00, aubreymoore9441
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1


Chemistry, 22.06.2019 11:40, Wemaybewrong
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1
You know the right answer?
What mass of pcl5 will be produced from the given masses of both reactants? 28.0 g of p4 and 59.0 g...
Questions in other subjects:

Arts, 24.03.2021 01:00


Mathematics, 24.03.2021 01:00


Geography, 24.03.2021 01:00



Mathematics, 24.03.2021 01:00

Computers and Technology, 24.03.2021 01:00

English, 24.03.2021 01:00

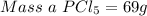
 will be produced from the given masses of both reactants? 28.0 g of P4 and 59.0 g of Cl2
will be produced from the given masses of both reactants? 28.0 g of P4 and 59.0 g of Cl2