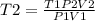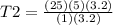Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, alaina3792
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 07:30, avisconti571
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

You know the right answer?
Calculate the final temperature (°c) of a gas if 32.0 l of the gas at 25°c and 1.00 atm is compresse...
Questions in other subjects:



Mathematics, 18.05.2021 18:50






Mathematics, 18.05.2021 18:50