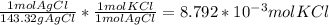Chemistry, 25.10.2019 00:43 cookiebrain72
Consider the following balanced chemical equation, kcl(aq) agno3(aq) → agcl(s) kno3(aq)when a sample of impure potassium chloride (0.900 g) was dissolved in water, and treated with excess silver nitrate (agno3), 1.26 g of silver chloride (agcl) was precipitated. calculate the percentage kcl in the original sample.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, strevino9178
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 23.06.2019 01:30, Michael845313
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1


Chemistry, 23.06.2019 06:40, donalyndearingbizz
15. what volume of cci, (d = 1.6 g/cc) contain6.02 x 1025 cci, molecules (ci = 35.5)(1) 10.5 l(2) 250 ml(3) 9.625 l(4) 1.712 lplz answer with step by step explanation
Answers: 1
You know the right answer?
Consider the following balanced chemical equation, kcl(aq) agno3(aq) → agcl(s) kno3(aq)when a sample...
Questions in other subjects:


Physics, 01.08.2019 17:00






History, 01.08.2019 17:00


Chemistry, 01.08.2019 17:00