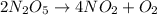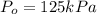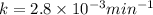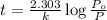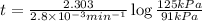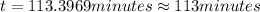The decomposition of dinitrogen pentoxide, n2o5, to no2 and o2 is a first-order reaction. at 60°c, the rate constant is 2.8 × 10-3min-1. if a rigid vessel initially contains only n2o5 at a pressure of 125 kpa, how long will it take for the total pressure to reach 176 kpa? options (pick 1)a)113 minb)129 minc)42 mind)182 mine)62 minf)83 min

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 21:30, artemiscrock77041
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1


Chemistry, 22.06.2019 17:10, sophiaa23
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1
You know the right answer?
The decomposition of dinitrogen pentoxide, n2o5, to no2 and o2 is a first-order reaction. at 60°c, t...
Questions in other subjects:


Social Studies, 21.07.2019 09:50

Mathematics, 21.07.2019 09:50


Mathematics, 21.07.2019 09:50