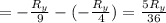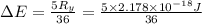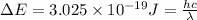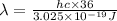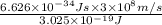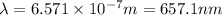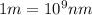The energy e of the electron in a hydrogen atom can be calculated from the bohr formula:
e= -ry/n^2
in this equation ry stands for the rydberg energy, and n stands for the principal quantum number of the orbital that holds the electron.
calculate the wavelength of the line in the absorption line spectrum of hydrogen caused by the transition of the electron from an orbital with n=2 to an orbital with n=3.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 01:00, deaishaajennings123
What is the equilibrium constant of aa+bb=cc+dd
Answers: 1
You know the right answer?
The energy e of the electron in a hydrogen atom can be calculated from the bohr formula:
Questions in other subjects:

Chemistry, 17.02.2021 21:30

Mathematics, 17.02.2021 21:30




Mathematics, 17.02.2021 21:40


Spanish, 17.02.2021 21:40


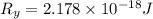
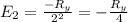
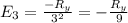
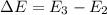 :
: