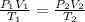Chemistry, 24.10.2019 17:43 dominaricann2451
The formula of nitrogen oxide is no, of nitrogen dioxide is no_2. write a balanced equation for the reaction of nitrogen oxide with oxygen. for each (1.0) mole of no: i. how many moles of o_2 are consumed and of no_2 produced? ii. how many liters of each (state conditions)? iii. how many grams of each? d. calculate the mass of 4.55 l of hci gas at stp. e. find the molecular mass of a gas having a density of a gas having a density of 0.00481 g/ml at stp. f. write a balanced equation for the reaction you caused to occur in this assignment. solve the following problems, assume that you used a sample with a mass of 2.550 grams. i. if the sample was pure kcio_3 and it was completely decomposed into kci and o_2, fill in the following blanks: there will be moles of o_2 obtained, having a mass of grams and occupying ml at stp or ml at 29 degree c and 732 torr. ii. assume now that the sample was an unknown mixture of kcio_3 with kci and that complete decomposition yielded 182 ml of oxygen gas measured at 29 degree c and 732 torr. calculate the percent by mass of kcio_3 in the mixture. g. nitrogen gas was collected over water at 26 degree c and 755 torr. if the volume collected was 35.9l. what volume would it occupy, dry, at stp?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, VictoriaRose520
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 08:00, katelyn0579
Straightforward questions answered in the powerpoint slidesreaction: heating the starting materials under refluxwhat does it mean to heat under reflux? why do we choose water as the reflux solvent? what are boiling chips used for? why do we put a condenser on top of the reaction? why do we add heat and let the reaction stir for 30 minutes? why do we add sulfuric acid to the reaction after it cools as opposed to when it’s still hot? separation: filtration of precipitatewhy don’t we do an aqueous and organic extraction in the separatory funnel? why do you rinse the salicylic acid on the filter with ice cold water? purification: recrystallization of salicylic acid (no hot filtration needed)what is the difference in the amount of room temperature water vs. boiling water needed to dissolve the salicylic acid (assume a 1.2 gram yield of salicylic acid)? remember, in the lab if you need x ml of boiling water to dissolve a solid, then you should add a little more (definitely no more than 1.5 times the theoretical amount) to ensure it doesn’t recrystallize prematurely. analysis: melting point of salicylic acidwhat can you conclude if the melting point of the salicylic acid you just synthesized is 152-155oc and the 1: 1 mix of your product and “synthetic” salicylic acid is 151-154oc?
Answers: 1

You know the right answer?
The formula of nitrogen oxide is no, of nitrogen dioxide is no_2. write a balanced equation for the...
Questions in other subjects:

English, 27.01.2021 01:00



Mathematics, 27.01.2021 01:00

Mathematics, 27.01.2021 01:00

Mathematics, 27.01.2021 01:00



Physics, 27.01.2021 01:00

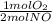 = 0,5 mol of O₂
= 0,5 mol of O₂ = 1 mol of NO₂
= 1 mol of NO₂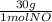 = 30g
= 30g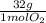 = 16g
= 16g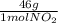 = 46g
= 46g = 7,41 g of HCl
= 7,41 g of HCl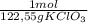 = 0,0208 moles of KClO₃
= 0,0208 moles of KClO₃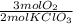 = 0,0312 moles of O₂
= 0,0312 moles of O₂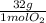 = 0,999g of O₂
= 0,999g of O₂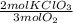 = 0,0106 mol KClO₃. In grams:
= 0,0106 mol KClO₃. In grams: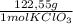 = 1,300 g of KClO₃.
= 1,300 g of KClO₃.