
Chemistry, 24.10.2019 10:43 sahramusa035
If the density of mercury is 13.6g/ml, what is the mass in grams of 3426 ml of the liquid?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, alexandroperez13
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 21.06.2019 22:00, pettygirl13
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3


Chemistry, 22.06.2019 08:30, Apple557
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1
You know the right answer?
If the density of mercury is 13.6g/ml, what is the mass in grams of 3426 ml of the liquid?...
Questions in other subjects:



Biology, 27.01.2021 05:50


Mathematics, 27.01.2021 05:50


Social Studies, 27.01.2021 05:50

Mathematics, 27.01.2021 05:50


Mathematics, 27.01.2021 05:50

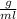
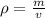 and mass can be derived as
and mass can be derived as
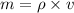
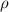 =density of the cylinder
=density of the cylinder


