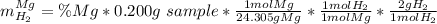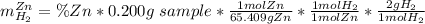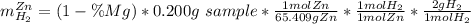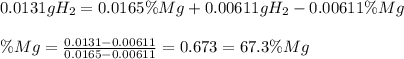Chemistry, 24.10.2019 04:00 sierravick123owr441
A0.200 g sample of magnesium and zinc is placed in a rigid 1 l vessel containing dry air at 25 °c and 1 atm. the magnesium and zinc are treated with dilute sulfuric acid to produce hydrogen gas according to the following balanced reactions: mg (s) + h2so4 (aq) → mg2+ (aq) + so42– (aq) + h2 (g) zn (s) + h2so4 (aq) → zn2+ (aq) + so42– (aq) + h2 (g) the hydrogen gas is then completely combusted in the same vessel to form water as a product. the vessel is then cooled back to 25 °c (assume that all the water condenses) and the pressure is found to be 0.95 atm. what is the mass percentage of magnesium in the mixture?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, madisonrosamond99
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 08:30, Apple557
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1


Chemistry, 22.06.2019 16:10, 00015746
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
A0.200 g sample of magnesium and zinc is placed in a rigid 1 l vessel containing dry air at 25 °c an...
Questions in other subjects:

Chemistry, 30.09.2019 04:30

History, 30.09.2019 04:30



Mathematics, 30.09.2019 04:30


Mathematics, 30.09.2019 04:30


Biology, 30.09.2019 04:30

English, 30.09.2019 04:30

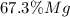
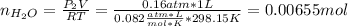
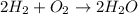
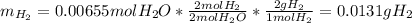
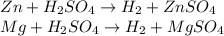
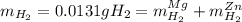
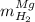 accounts for the hydrogen yielded by the magnesium and
accounts for the hydrogen yielded by the magnesium and 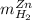 by the zinc which are computed in terms of the stoichiometry and the initial sample's composition as shown below:
by the zinc which are computed in terms of the stoichiometry and the initial sample's composition as shown below: