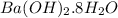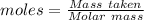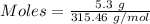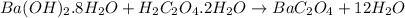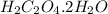Chemistry, 24.10.2019 03:00 austinmontgomep7foxp
Barium oxalate is used as a colorant to produce the green color in fireworks. imagine that you have been assigned to prepare barium oxalate by reacting barium hydroxide octahydrate (ba(oh)2·8h2o) with oxalic acid dihydrate (h2c2o4·2h2o). find the number of grams of oxalic acid dihydrate that would be required to completely react with 5.3 g of barium hydroxide octahydrate. use correct significant figures. do not include a unit with your answer or it will be counted wrong.
h2c2o4·2h2o 126.07 g/mol
h2c2o4 90.03 g/mol
ba(oh)2·8h2o 315.46 g/mol
ba(oh)2 171.34 g/mol

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, shaylawaldo11
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

Chemistry, 22.06.2019 12:30, Svetakotok
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2
You know the right answer?
Barium oxalate is used as a colorant to produce the green color in fireworks. imagine that you have...
Questions in other subjects:

History, 22.06.2019 05:30

History, 22.06.2019 05:30


History, 22.06.2019 05:30




Mathematics, 22.06.2019 05:30

Mathematics, 22.06.2019 05:30

Physics, 22.06.2019 05:30