
Chemistry, 23.10.2019 23:00 itzygutierrez2763
8.5 ml of a 0.53 m solution of the strong acid hcl was diluted with water to a total volume of 1000.0 ml at 30°c. calculate the following. (the kw at 30°c is 1.46 ✕ 10−14.)
(a) the concentration of h3o+ ions in the diluted hcl solution m
(b) the ph of the diluted solution (c) the poh of the diluted solution

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:20, skyemichellec
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3

Chemistry, 22.06.2019 21:30, liamgreene90
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3

Chemistry, 23.06.2019 00:00, kittenalexis68
How many atoms or molecules are there in a mole of a substance?
Answers: 1
You know the right answer?
8.5 ml of a 0.53 m solution of the strong acid hcl was diluted with water to a total volume of 1000....
Questions in other subjects:

Mathematics, 08.06.2021 02:20


Mathematics, 08.06.2021 02:20

Mathematics, 08.06.2021 02:20



Arts, 08.06.2021 02:20


English, 08.06.2021 02:20

Geography, 08.06.2021 02:20

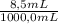 = 4,5x10⁻³ M of HCl
= 4,5x10⁻³ M of HCl

