
Chemistry, 23.10.2019 20:00 hetdashadia123
Ethanol, c2h5oh, is produced industrially from ethylene, c2h4, by the following sequence of reactions: 3c2h4 + 2h2so4 → c2h5hso4 + (c2h5)2so4 c2h5hso4 + (c2h5)2so4 + 3h2o → 3c2h5oh + 2h2so4 what volume of ethylene at stp is required to produce 1.000 metric ton (1000 kg) of ethanol if the overall yield of ethanol is 90.1%

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:30, lizzzzi7908
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1

Chemistry, 23.06.2019 02:00, hermesrobles
Which would freeze at a higher temperature: the great salt lake or lake tahoe? a. lake tahoe would freeze at a higher temperature. b. the great salt lake would freeze at a higher temperature. c. both lakes would freeze at the same temperature.
Answers: 2

Chemistry, 23.06.2019 06:00, kristine2424
How does a coronal mass ejection (cme) affect the solar wind? a cme adds more particles to the solar wind, intensifying it. a cme blocks the solar wind, causing it to fade. a cme does not affect the solar wind but it does affect auroras. a cme increases the amount of energy in the solar wind.
Answers: 2
You know the right answer?
Ethanol, c2h5oh, is produced industrially from ethylene, c2h4, by the following sequence of reaction...
Questions in other subjects:

Mathematics, 28.10.2020 07:00



Health, 28.10.2020 07:00



Mathematics, 28.10.2020 07:00

Business, 28.10.2020 07:00


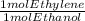 * 100/90.1 = 2.4128* 10⁴ mol ethylene
* 100/90.1 = 2.4128* 10⁴ mol ethylene


