
Chemistry, 23.10.2019 19:30 griffislandon74
A5.012-g sample of an iron chloride hydrate was dried in an oven. the mass of the anhydrous compound was 3.195 g. the compound was then dissolved in water and reacted with an excess of agno3. the agcl precipitate formed weighed 7.225 g. what is the formula of the original compound?

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 01:00, breemills9552
What two factors can affect the properties of a hydrocarbon? a. the number of its carbon atoms and the number of single bonds b. the number of its carbon atoms and the arrangement of its atoms c. the arrangement of its atoms and the number of its double bonds
Answers: 1
You know the right answer?
A5.012-g sample of an iron chloride hydrate was dried in an oven. the mass of the anhydrous compound...
Questions in other subjects:




Mathematics, 12.10.2020 14:01

History, 12.10.2020 14:01

Mathematics, 12.10.2020 14:01


English, 12.10.2020 14:01

History, 12.10.2020 14:01

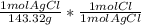 = 0.05041 mol Cl0.05041 mol Cl * 35.45 g/mol = 1.787 g Cl
= 0.05041 mol Cl0.05041 mol Cl * 35.45 g/mol = 1.787 g Cl


