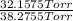Chemistry, 23.10.2019 19:20 SavgeKid7578
Adilute solution of bromine in carbon tetrachloride behaves as an ideal-dilute solution. the vapour pressure of pure ccl4 is 33.85 torr at 298k. the henry’s law constant when the concentration of br2 is expressed as a mole fraction is 122.36 torr. calculate the vapour pressure of each component, the total pressure, and the composition of the vapour phase when the mole fraction of br2 is 0.050, on the assumption that the conditions of the ideal-dilute solution are satisfied at this condition

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, emfranco1
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 21:30, emmalucilleblaha1995
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1


Chemistry, 23.06.2019 00:30, motorxr714
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1
You know the right answer?
Adilute solution of bromine in carbon tetrachloride behaves as an ideal-dilute solution. the vapour...
Questions in other subjects:




Mathematics, 26.02.2021 04:20

English, 26.02.2021 04:20

English, 26.02.2021 04:20

Chemistry, 26.02.2021 04:20

Mathematics, 26.02.2021 04:20

English, 26.02.2021 04:20

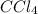 = 33.85 Torr
= 33.85 Torr = 122.36 torr
= 122.36 torr