
The sulfur content of an ore is determined gravimetrically by reacting the ore with concentrated nitric acid and potassium chlorate, converting all sulfur to sulfate. the excess nitrate and chlorate is removed by reaction with concentrated hydrochloric acid and the sulfate is precipitated using barium cation.
ba2+ (aq) + so42- (aq) = baso4 (s)
analysis of 12.3430 grams of a sulfur containing ore yielded 12.5221 grams of baso4. what is the percent by mass sulfur in the ore? (baso4 = 233.43 g/mol). show all calculation.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, rebeccacruzz2017
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3


You know the right answer?
The sulfur content of an ore is determined gravimetrically by reacting the ore with concentrated nit...
Questions in other subjects:

Chemistry, 22.07.2021 06:30



Engineering, 22.07.2021 06:30

Mathematics, 22.07.2021 06:30





Mathematics, 22.07.2021 06:30

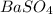 = 12.5221 g
= 12.5221 g
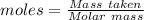
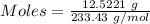
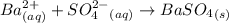
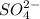
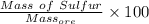 =
= 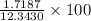 = 13.92 %
= 13.92 %

