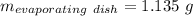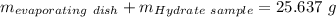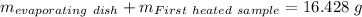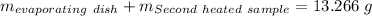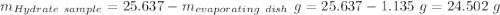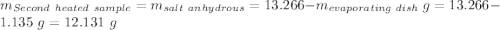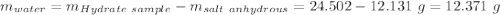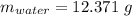Astudent heats a sample of hydrate once, and the mass of the sample and the evaporating dish is 16.428 g. after a second heating cycle, the mass of the sample and the dish is 13.266 g. the student stops the heat/cool/weigh cycle after the second time. if the original mass of the evaporating dish and the hydrate was 25.637 g, and the mass of the evaporating dish alone was 1.135 g, what is the mass of water removed from the sample by heating? provide your response to three digits after the decimal.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, naomicervero
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2


Chemistry, 22.06.2019 16:10, 00015746
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
Astudent heats a sample of hydrate once, and the mass of the sample and the evaporating dish is 16.4...
Questions in other subjects:

English, 26.01.2021 21:30

Mathematics, 26.01.2021 21:30

Arts, 26.01.2021 21:30

Geography, 26.01.2021 21:30

Mathematics, 26.01.2021 21:30


Mathematics, 26.01.2021 21:30