
He chlorination of methane occurs in a number of steps that results in the formation of chloromethane and hydrogen chloride. the overall reaction is 2ch4(g)+3cl2(g)⟶2ch3cl(g)+2hcl(g)+2 cl−(g) 2ch4(g)+3cl2(g)⟶2ch3cl(g)+2hcl(g)+2 cl−(g) suppose that a chemist combines 295 ml295 ml of methane and 725 ml725 ml of chlorine at stp in a 2.00 l2.00 l flask. the flask is then allowed to stand at 298 k.298 k. if the reaction reaches 77.0%77.0% completion, what is the total pressure in the flask?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, sammysosa121832
The ph of carrots are 5.0 how it is classified a. acidic b. basic c. indicator d. neutral
Answers: 2

Chemistry, 21.06.2019 21:00, advancedgamin8458
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2


Chemistry, 22.06.2019 10:00, melissa9882
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1
You know the right answer?
He chlorination of methane occurs in a number of steps that results in the formation of chloromethan...
Questions in other subjects:




Mathematics, 28.06.2019 16:00





Mathematics, 28.06.2019 16:00

Mathematics, 28.06.2019 16:00

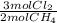 = 0,0198 moles Cl₂. That means that 0,0324-0,0198 = -0,0126 moles of Cl₂ are in excess-.
= 0,0198 moles Cl₂. That means that 0,0324-0,0198 = -0,0126 moles of Cl₂ are in excess-. = 0,0102 moles of CH₃Cl -that are the same moles of HCl and Cl⁻-
= 0,0102 moles of CH₃Cl -that are the same moles of HCl and Cl⁻-

