
Chemistry, 23.10.2019 18:00 krishawnnn
For the generic equilibrium ha(aq) ⇌ h+(aq) + a−(aq), which of these statements is true? for the generic equilibrium , which of these statements is true? if you add the soluble salt ka to a solution of ha that is at equilibrium, the concentration of ha would decrease. if you add the soluble salt ka to a solution of ha that is at equilibrium, the ph would increase. the equilibrium constant for this reaction changes as the ph changes. if you add the soluble salt ka to a solution of ha that is at equilibrium, the concentration of a− would decrease.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:30, kaytonleeb
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 22:30, needhelpasap8957
Why is the bottom layer of a trophic pyrimid the
Answers: 2


Chemistry, 22.06.2019 23:30, Xavier8247
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3
You know the right answer?
For the generic equilibrium ha(aq) ⇌ h+(aq) + a−(aq), which of these statements is true? for the ge...
Questions in other subjects:

Mathematics, 04.08.2019 09:30




Biology, 04.08.2019 09:30

Mathematics, 04.08.2019 09:30




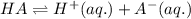
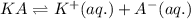
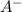 ion is getting increased on the product side, so the equilibrium will shift in the direction to minimize this effect, which is in the direction of HA.
ion is getting increased on the product side, so the equilibrium will shift in the direction to minimize this effect, which is in the direction of HA.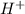 ions are getting decreases. This will increase the pH of the solution.
ions are getting decreases. This will increase the pH of the solution.

