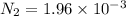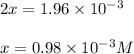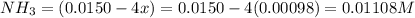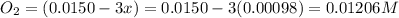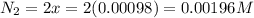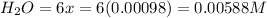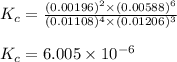Chemistry, 23.10.2019 17:00 tasnimabdallah971
Be sure to answer all parts. the first step in hno3 production is the catalyzed oxidation of nh3. without a catalyst, a different reaction predominates: 4 nh3(g) +3 o2 (g) ⇌ 2 n2(g) + 6 h2o(g) when 0.0150 mol of nh3(g) and 0.0150 mol of o2(g) are placed in a 1.00−l container at a certain temperature, the n2 concentration at equilibrium is 1.96 × 10−3 m. calculate kc.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, granthazenp5e9mj
Which feature do highland climates have that lower elevation areas do not?
Answers: 1


Chemistry, 22.06.2019 14:20, greenbyron88
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 19:30, Karinaccccc
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1
You know the right answer?
Be sure to answer all parts. the first step in hno3 production is the catalyzed oxidation of nh3. wi...
Questions in other subjects:


History, 21.08.2019 08:10




Physics, 21.08.2019 08:10




Mathematics, 21.08.2019 08:10

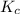 for the reaction is
for the reaction is 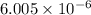
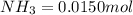
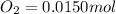
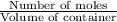
![[NH_3]_i=\frac{0.0150}{1.00}=0.0150M](/tpl/images/0342/7531/438ea.png)
![[O_2]_i=\frac{0.0150}{1.00}=0.0150M](/tpl/images/0342/7531/0aac8.png)
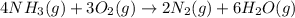
![K_c=\frac{[N_2]^2[H_2O]^6}{[NH_3]^4[O_2]^3}](/tpl/images/0342/7531/b62d0.png) .......(1)
.......(1)