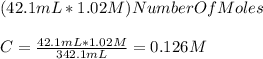Chemistry, 23.10.2019 17:00 ahmedislife
If 42.1 ml of 1.02 m sodium hydroxide, measured using a graduated cylinder, is placed in a beaker filled with 300 ml of di water, what is the concentration of the diluted naoh solution?
b) why does this calculation only provide an estimate of the naoh concentration? in other words, why do we have to standardize the naoh in this experiment to find its exact concentration?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, rosie20052019
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 02:10, board1692
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 16:50, TheOriginal2x
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 22.06.2019 21:30, rileydavidharless
Which substance can be broken down by chemical means
Answers: 1
You know the right answer?
If 42.1 ml of 1.02 m sodium hydroxide, measured using a graduated cylinder, is placed in a beaker fi...
Questions in other subjects:

Mathematics, 20.09.2020 02:01


History, 20.09.2020 02:01

Mathematics, 20.09.2020 02:01


Mathematics, 20.09.2020 02:01

Mathematics, 20.09.2020 02:01