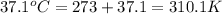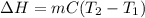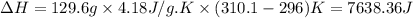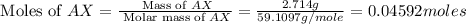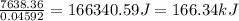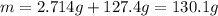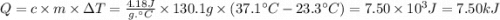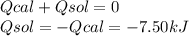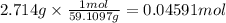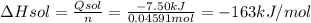Chemistry, 23.10.2019 17:00 mrgandollins5222
When 2.714 g of ax (s) dissolves in 127.4 g of water in a coffee-cup calorimeter the temperature rises from 23.3 °c to 37.1 °c. calculate the enthalpy change (in kj/mol) for the solution process. ax(s) → a+(aq) + x-(aq) assumptions for this calculation: the specific heat of the solution is the same as that of pure water (4.18 j/gk) the density of water = 1.000 g/ml the liquid’s final volume is not changed by adding the solid the calorimeter loses only a negligible quantity of heat. the formula weight of ax = 59.1097 g/mol. be sure you include the correct sign for the enthalpy change.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:10, josephpezza18
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1



Chemistry, 23.06.2019 01:30, Elliendc7939
List and describe the neurological effects of the vocs and other air pollutants, as described by dr. theo colborn
Answers: 2
You know the right answer?
When 2.714 g of ax (s) dissolves in 127.4 g of water in a coffee-cup calorimeter the temperature ris...
Questions in other subjects:

Mathematics, 23.03.2021 01:00


Mathematics, 23.03.2021 01:00

Mathematics, 23.03.2021 01:00

Geography, 23.03.2021 01:00



Biology, 23.03.2021 01:00


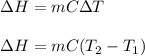
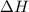 = change in enthalpy = ?
= change in enthalpy = ?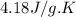
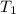 = initial temperature =
= initial temperature = 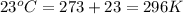
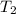 = final temperature =
= final temperature =