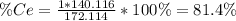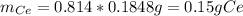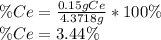Chemistry, 23.10.2019 16:50 chelseychew32
To find the ce4+ content in a solid sample, 4.3718 g of the solid sample were dissolved and treated with excess iodate to precipitate the ce4+ as ce(io3)4. the precipitate was collected, washed well, dried, and ignited to produce 0.1848 g of ceo2 (fm 172.114). what was the weight percentage of ce (am 140.116) in the original sample?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 03:30, HalpMahOnMahH0meW0rk
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1

Chemistry, 23.06.2019 04:20, tyrickdavis1
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1

Chemistry, 23.06.2019 04:31, 24swimdylanoh
What are the coefficients that will balance the skeleton equation below? n2 + h2 → nh3
Answers: 1
You know the right answer?
To find the ce4+ content in a solid sample, 4.3718 g of the solid sample were dissolved and treated...
Questions in other subjects:


French, 23.11.2021 17:10



Biology, 23.11.2021 17:10



Mathematics, 23.11.2021 17:20


Social Studies, 23.11.2021 17:20

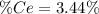
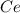 into the
into the 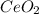 by using their respective molar masses as shown below:
by using their respective molar masses as shown below: