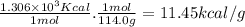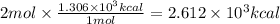Combustion reactions are exothermic. the heat of reaction for the combustion of 2-methylheptane, c8h18, is 1.306×103 kcal/mol. what is the heat of combustion for 2-methylheptane in kcal/gram? 11.45 kcal/gram how much heat will be given off if molar quantities of 2-methylheptane react according to the following equation? 2 c8h18 + 25 o216 co2 + 18 h2o

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:00, bobbycisar1205
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2


Chemistry, 22.06.2019 17:10, hahahwha
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
You know the right answer?
Combustion reactions are exothermic. the heat of reaction for the combustion of 2-methylheptane, c8h...
Questions in other subjects:

Mathematics, 07.12.2021 20:30

Mathematics, 07.12.2021 20:30

Mathematics, 07.12.2021 20:30

Mathematics, 07.12.2021 20:30


English, 07.12.2021 20:30



Mathematics, 07.12.2021 20:30

Mathematics, 07.12.2021 20:30