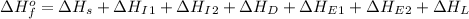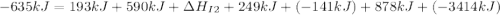Use the data given below to construct a born-haber cycle to determine the second ionization energy of ca. δ h°(kj) ca(s)→ca(g) 193 ca(g)→ca (g) e− 590 2o(g)→o2(g) - 498 o(g) e−→o−(g) - 141 o−(g) e−→o2−(g) 878 ca(s) 12o2(g)→cao(s) - 635 ca2 (g) o2−(g)→cao(s) - 34142o(g) > o2(g) - 498o(g) + e- > o-(g) - 141o-(g) + e- > o2-(g) 878ca(s) + 1/2 o2(g) > cao(s) - 635

Answers: 1


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 09:30, crystalhoff9419
My plate recommends that of your nutritional intake comes from fruits and vegetables. a. 30% b. 50% c. 20% d. 40%
Answers: 2
You know the right answer?
Use the data given below to construct a born-haber cycle to determine the second ionization energy o...
Questions in other subjects:





Health, 24.10.2021 04:10



Mathematics, 24.10.2021 04:10


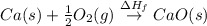
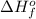 = enthalpy of formation of calcium oxide = -635 kJ
= enthalpy of formation of calcium oxide = -635 kJ :
: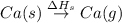
 = sublimation energy of calcium = 193 kJ
= sublimation energy of calcium = 193 kJ
 = first ionization energy of calcium = 590 kJ
= first ionization energy of calcium = 590 kJ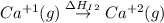
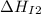 = second ionization energy of calcium = ?
= second ionization energy of calcium = ?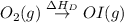

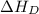 = dissociation energy of oxygen =
= dissociation energy of oxygen = 

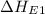 = first electron affinity energy of oxygen = -141 kJ
= first electron affinity energy of oxygen = -141 kJ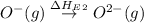
 = second electron affinity energy of oxygen = 878 kJ
= second electron affinity energy of oxygen = 878 kJ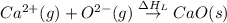
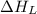 = lattice energy of calcium oxide = -3414 kJ
= lattice energy of calcium oxide = -3414 kJ