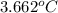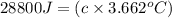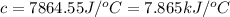Chemistry, 23.10.2019 02:00 michaellangley
The enthalpy of combustion of benzoic acid (c6h5cooh) which is often used to calibrate calorimeters, is −3227 kj/mol. when 1.09 g of benzoic acid was burned in a calorimeter, the temperature increased by 3.662◦c. what is the overall heat capacity of the calorimeter? the overall heat capacity includes the calorimeter hardware and the water that is in it. answer in units of kj/ ◦c.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:50, hannahmyung1113
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3


Chemistry, 23.06.2019 09:00, blossie94681
The vapor pressure of water at 25.0°c is 23.8 torr. determine the mass of glucose (molar mass = 180 g/mol) needed to add to 500.0 g of water to change the vapor pressure to 22.8 torr.
Answers: 1
You know the right answer?
The enthalpy of combustion of benzoic acid (c6h5cooh) which is often used to calibrate calorimeters,...
Questions in other subjects:

Mathematics, 19.10.2019 11:30

Health, 19.10.2019 11:30

Mathematics, 19.10.2019 11:30


Mathematics, 19.10.2019 11:30


Mathematics, 19.10.2019 11:30

Social Studies, 19.10.2019 11:30


Biology, 19.10.2019 11:30



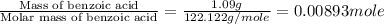
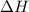 = enthalpy of combustion = 3227 kJ/mole
= enthalpy of combustion = 3227 kJ/mole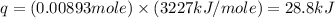
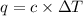
 = heat capacity of calorimeter = ?
= heat capacity of calorimeter = ? = change in temperature =
= change in temperature =