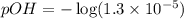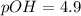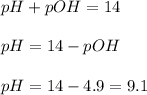Chemistry, 23.10.2019 01:00 anthonybowie99
Lithium acetate, lich3co2, is a salt formed from the neutralization of the weak acid acetic acid, ch3co2h, with the strong base lithium hydroxide. given that the value of ka for acetic acid is 1.8×10−5, what is the ph of a 0.289 m solution of lithium acetate at 25∘c?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, coryoddoc3685
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2


Chemistry, 22.06.2019 18:00, brisacruz013
Which statement best describes the he properties of iconic compounds ?
Answers: 1
You know the right answer?
Lithium acetate, lich3co2, is a salt formed from the neutralization of the weak acid acetic acid, ch...
Questions in other subjects:


History, 02.07.2019 00:00


Mathematics, 02.07.2019 00:00

Mathematics, 02.07.2019 00:00

Mathematics, 02.07.2019 00:00


Social Studies, 02.07.2019 00:00


Spanish, 02.07.2019 00:00

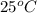 is 9.1
is 9.1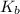 .
.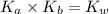
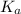 = dissociation constant of an acid =
= dissociation constant of an acid = 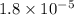
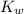 = dissociation constant of water =
= dissociation constant of water = 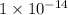
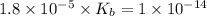
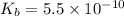
![[OH^-]=(K_b\times C)^{\frac{1}{2}}](/tpl/images/0341/6745/115e0.png)
![[OH^-]=(5.5\times 10^{-10}\times 0.289)^{\frac{1}{2}}](/tpl/images/0341/6745/5aa0e.png)
![[OH^-]=1.3\times 10^{-5}M](/tpl/images/0341/6745/43771.png)
![pOH=-\log [OH^-]](/tpl/images/0341/6745/1fac1.png)