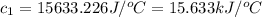Chemistry, 23.10.2019 00:00 josephbrowne9p18dit
The enthalpy of combustion of benzoic acid (c6h5cooh) is commonly used as the standard for calibrating constant-volume bomb calorimeters; its value has been accurately determined to be −3226.7 kj/mol. when 2.8161 g of benzoic acid are burned in a calorimeter, the temperature rises from 21.84°c to 24.67°c. what is the heat capacity of the bomb? (assume that the quantity of water surrounding the bomb is exactly 2250 g.)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, akeemedwards12
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2

Chemistry, 22.06.2019 16:50, briansalazar17
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3


Chemistry, 23.06.2019 01:00, bsheepicornozj0gc
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
You know the right answer?
The enthalpy of combustion of benzoic acid (c6h5cooh) is commonly used as the standard for calibrati...
Questions in other subjects:





Biology, 27.06.2019 05:00

Chemistry, 27.06.2019 05:00

Chemistry, 27.06.2019 05:00

Mathematics, 27.06.2019 05:00

Biology, 27.06.2019 05:00

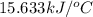
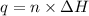
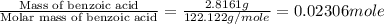
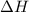 = enthalpy of combustion = 3226.7 kJ/mole
= enthalpy of combustion = 3226.7 kJ/mole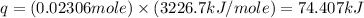
![q=[q_1+q_2]](/tpl/images/0341/5440/341bc.png)
![q=[c_1\times \Delta T+m_2\times c_2\times \Delta T]](/tpl/images/0341/5440/1d50b.png)
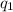 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter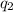 = heat absorbed by the water
= heat absorbed by the water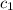 = specific heat of calorimeter = ?
= specific heat of calorimeter = ?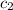 = specific heat of water =
= specific heat of water = 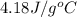
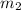 = mass of water = 2550 g
= mass of water = 2550 g = change in temperature =
= change in temperature = 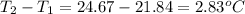
![74407J=[(c_1\times 2.83^oC)+(2550g\times 4.18J/g^oC\times 2.83^oC)]](/tpl/images/0341/5440/a2494.png)