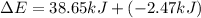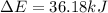Chemistry, 23.10.2019 00:30 kandigirl9990
Limestone stalactites and stalagmites are formed in caves by the following reaction: ca2 (aq) 2hco−3(aq)→caco3(s) co2(g) h2o(l) if 1 mol of caco3 forms at 298 k under 1 atm pressure, the reaction performs 2.47 kj of p−v work, pushing back the atmosphere as the gaseous co2 forms. at the same time, 38.65 kj of heat is absorbed from the environment. what is the value of δe for this reaction

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, mazielynn84
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 10:10, dhailyortegacampa131
Stage in which a typical star has completely stopped fusion
Answers: 1


Chemistry, 22.06.2019 11:00, jodonw5616
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1
You know the right answer?
Limestone stalactites and stalagmites are formed in caves by the following reaction: ca2 (aq) 2hco−...
Questions in other subjects:

Mathematics, 13.01.2021 04:20


History, 13.01.2021 04:20







History, 13.01.2021 04:20

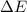 for this reaction is 36.18 kJ
for this reaction is 36.18 kJ