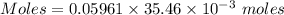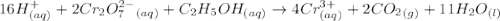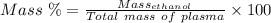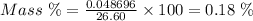Aperson's blood alcohol (c2h5oh) level can be determined by titrating a sample of blood plasma with a potassium dichromate solution. the balanced equation is 16h (aq) 2cr2o72−(aq) c2h5oh(aq) → 4cr3 (aq) 2co2(g) 11h2o(l) if 35.46 ml of 0.05961 m cr2o72− is required to titrate 26.60 g of plasma, what is the mass percent of alcohol in the blood?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, hellokitty1647
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i. e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 01:00, AIhunter2884
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 23.06.2019 02:00, Paytonsmommy09
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2

Chemistry, 23.06.2019 06:00, mustafajibawi1
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes? or no? this question is worth 20 points! let it be correct!
Answers: 1
You know the right answer?
Aperson's blood alcohol (c2h5oh) level can be determined by titrating a sample of blood plasma with...
Questions in other subjects:

Biology, 07.04.2020 03:03

Mathematics, 07.04.2020 03:03

Mathematics, 07.04.2020 03:03

Mathematics, 07.04.2020 03:03



Biology, 07.04.2020 03:03



Health, 07.04.2020 03:03

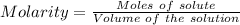
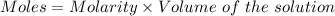
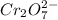 :
: