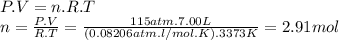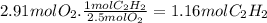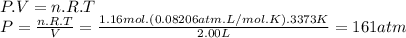Chemistry, 22.10.2019 23:00 kihgff5711
Magine that you have a 7.00 l gas tank and a 2.00 l gas tank. you need to fill one tank with oxygen and the other with acetylene to use in conjunction with your welding torch. if you fill the larger tank with oxygen to a pressure of 115 atm , to what pressure should you fill the acetylene tank to ensure that you run out of each gas at the same time? assume ideal behavior for all gases.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:20, kevinhernandez582
What will most likely happen when two bromine atoms bond together?
Answers: 3

You know the right answer?
Magine that you have a 7.00 l gas tank and a 2.00 l gas tank. you need to fill one tank with oxygen...
Questions in other subjects:

Mathematics, 09.06.2021 15:30

Mathematics, 09.06.2021 15:30

Mathematics, 09.06.2021 15:30

Biology, 09.06.2021 15:30


Mathematics, 09.06.2021 15:30

Mathematics, 09.06.2021 15:30

Mathematics, 09.06.2021 15:30