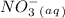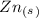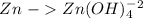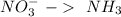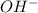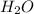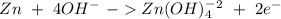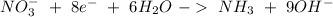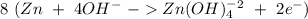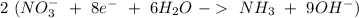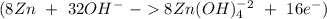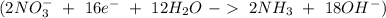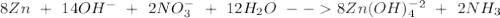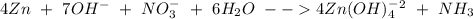Chemistry, 22.10.2019 21:00 jjimenez0276
Balance the following skeleton reaction and identify the oxidizing and reducing agents: include the states of all reactants and products in your balanced equation. you do not need to include the states with the identities of the oxidizing and reducing agents. zn(s) + no3−(aq) → zn(oh)42−(aq) + nh3(g) [basic]

Answers: 1


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 12:10, coastieltp58aeg
Building glycogen from glucose molecules is an example of
Answers: 3
You know the right answer?
Balance the following skeleton reaction and identify the oxidizing and reducing agents: include the...
Questions in other subjects:

Mathematics, 22.01.2021 23:00

Chemistry, 22.01.2021 23:00


Mathematics, 22.01.2021 23:00



Biology, 22.01.2021 23:00

Arts, 22.01.2021 23:00

History, 22.01.2021 23:00

English, 22.01.2021 23:00