Chemistry, 22.10.2019 20:00 destinyaus14
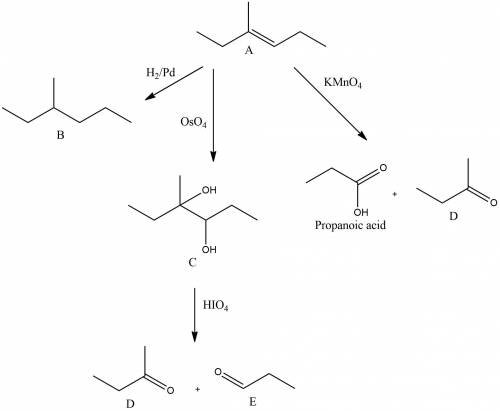
An unknown hydrocarbon a with the formula c7h14 reacts with 1 molar equivalent of h2 over a palladium catalyst to give hydrocarbon b. hydrocarbon a also reacts with oso4 to give diol c. treatment of diol c with periodic acid gives ketone d and aldehyde e. when oxidized with kmno4 in acidic solution, a gives two fragments. one fragment is propionic acid, ch3ch2co2h, and the other fragment is ketone d. draw the structure of compound d.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:30, lasagnafoe
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 14:50, wcraig1998
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1
You know the right answer?
An unknown hydrocarbon a with the formula c7h14 reacts with 1 molar equivalent of h2 over a palladiu...
Questions in other subjects:


World Languages, 01.02.2020 00:59

Geography, 01.02.2020 00:59

Mathematics, 01.02.2020 00:59





Physics, 01.02.2020 00:59

 .The hydrocarbon A should contain only one double bond as it reacts with 1 molar equivalent of
.The hydrocarbon A should contain only one double bond as it reacts with 1 molar equivalent of  over palladium.The hydrocarbon A contains seven carbon atom. Therefore the ketone D should contain 4 carbon atoms as propionic acid ( 3 carbon atom) is produced as another fragment.Reaction and structure of D has been shown below.
over palladium.The hydrocarbon A contains seven carbon atom. Therefore the ketone D should contain 4 carbon atoms as propionic acid ( 3 carbon atom) is produced as another fragment.Reaction and structure of D has been shown below.