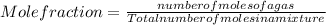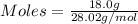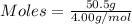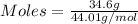Chemistry, 22.10.2019 02:00 jstringe424
Asample contains 18.0 g n2 (mw = 28.02 g/mol), 50.5 g he (mw = 4.00 g/mol), and 34.6 g co2 (mw = 44.01 g/mol). calculate the mole fraction of carbon dioxide in the sample.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:30, abdullaketbi71
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1



Chemistry, 23.06.2019 06:50, isabellainksow87vn
The student repeated the experiment using a higher concentration of acid. the same volume of acid and the same mass of magnesium ribbon were used. what volume of hydrogen gas would have been produced after 60 seconds?
Answers: 1
You know the right answer?
Asample contains 18.0 g n2 (mw = 28.02 g/mol), 50.5 g he (mw = 4.00 g/mol), and 34.6 g co2 (mw = 44....
Questions in other subjects:

Mathematics, 30.11.2020 14:00

Mathematics, 30.11.2020 14:00

Social Studies, 30.11.2020 14:00

SAT, 30.11.2020 14:00


Business, 30.11.2020 14:00